Participation in these studies must be in compliance with your local ethical and patient consent guidelines.
Covid-19 Serum Studies
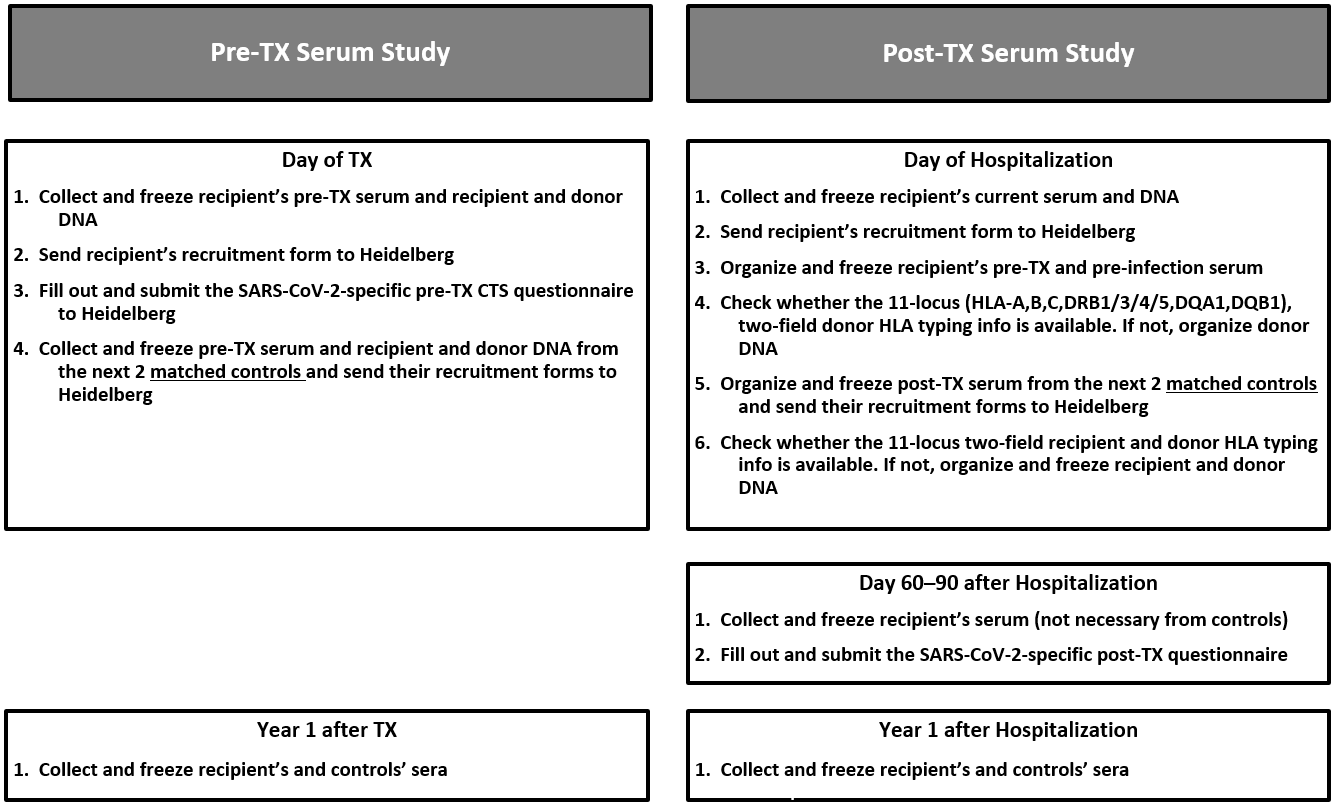
In the Pre-Transplant Covid-19 Serum Study we will measure in transplant recipients with a history of SARS-CoV-2 infection and the next two transplanted control patients matched for deceased or living donor and, if possible, also for age and gender, the DSA formation during the first post-transplant year. One pre- and one post-transplant serum and, for reliable determination of DSA, recipient and donor DNA will be required from all recruited patients. Furthermore, we will ask you to fill out a short SARS-CoV-2-specific pre-transplant questionnaire. Additional information will be deducted from regular CTS forms.
In the Post-Transplant Covid-19 Serum Study we will determine the DSA formation in transplant recipients hospitalized for SARS-CoV-2 infection and two control patients matched for donor type and time interval after transplantation. Three sera are required â the first on the day of hospitalization, the second on day 60 (not necessary in controls) and the third at year 1 after hospitalization. In addition and if available, a pre-infection and a pre-transplant serum will be needed for the precise evaluation of de novo DSA development. If donor DNA should not be available, we will ask you in antibody-positive cases to report the two-field 11-loci HLA typing of the donor. On day 60 after hospitalization, you will receive a SARS-CoV-2-specific questionnaire. Additional information will be deducted again from regular CTS forms.
Both studies are restricted to 18â75-year-old first renal-only transplantations from deceased or living donors and full-size liver-only transplantations from deceased donors. If sufficient case numbers can be reached, we will restrict the recruitment to the period until December 15, 2021.
Serum samples of recipient
1â2 ml serum without additives. Two hours after blood donation, the serum should be separated from cell material by centrifugation and frozen and stored at -20°C.
DNA samples of recipient and donor
10 ml EDTA blood, 50 μg isolated DNA, buffy coat, or in the case of donor also spleen tissue can be frozen and stored at -20°C. If material for donor DNA is not available, his/her full two-field 11-loci HLA typing is required.
Shipment of frozen samples to Heidelberg, Germany
March 29/30, 2021; October 18, 2021; February 7, 2022
(Download Study Protocol) (Patient Information) (Consent Forms)Documentation
- SARS-CoV-2-specific questionnaires
- pre-TX Study (to be filled out on day of TX)
- post-TX Study (to be filled out on day 60â90 after hospitalization)
- Regular CTS forms
Procedure
Pre-TX Covid-19 Serum Study
In total one pre-TX and one post-TX serum and recipient and donor DNA from:
- 18â75-year-old patients with a history of SARS-CoV-2 infection who received a kidney-only or full-size liver-only TX between December 10, 2020 and December 15, 2021 and
- the next two SARS-CoV-2 Ab-negative transplanted controls matched for type of donor (living or deceased) and, if possible, also for gender and age category (1)
| Serum/Data Collection Scheme | |||
| Day of TX | Post-TX Year 1 | ||
|
|||
Post-TX Covid-19 Serum Study
In total three/five serum samples and recipient and donor DNA from:- 18â75-year-old kidney-only or full-size liver-only TX recipients who are hospitalized for SARS-CoV-2 infection between December 10, 2020 and December 15, 2021 and
- two transplanted controls without SARS-CoV-2 infection matched for donor type, time after TX (4), age category (1) and gender
| Serum/Data Collection Scheme | |||
| Day of Hospitalization for SARS-CoV-2 Infection | Day 60â90 after Hospitalization | Year 1 after Hospitalization (5) | |
|
|
||
- Age categories: 18â34, 35â59, 60â75
- 1â2 ml serum without additives
- 10 ml frozen EDTA-blood, spleen, buffy coat, lymphocytes or 50 μg isolated DNA
- 40â180, 181â365, 366â1095, 1096â2555 and >2555 days after TX
- 300â430 days after hospitalization
- Not required in controls
- 10 ml frozen EDTA-blood, spleen, buffy coat or 50 μg isolated DNA; if donor DNA is not available, full two-field 11-loci HLA typing of the donor
Day of recruitment: Please report the patient to Heidelberg using the organ and study-specific 'CTS Covid-19 Study Recruitment Forms' (download kidney form, liver form) and start collecting and freezing the samples at â20°C.